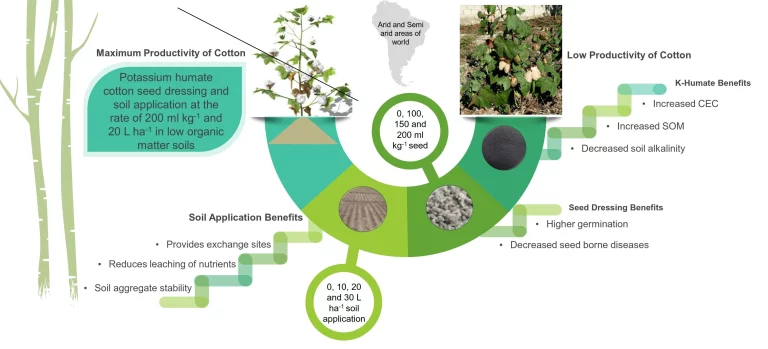
Difference Between Delta G and Delta G Naught
Key takeaways
- Delta G is the change in Gibbs free energy at any conditions, while delta G naught is the change in Gibbs free energy change at standard conditions (e.g. 298K and 1 atm).
- Delta G is used to predict the spontaneity of a reaction, while delta G naught is used to calculate the equilibrium constant of a reaction.
- Delta G naught is always negative for spontaneous reactions, and zero for reactions at equilibrium.
- The difference between delta G and delta G naught is the change in Gibbs free energy due to non-standard conditions, such as changes in concentration, pressure, or temperature.
Introduction
Gibbs free energy is a thermodynamic function that measures the amount of useful work that can be obtained from a system at constant temperature and pressure. It is denoted by the symbol G. The change in Gibbs free energy for a chemical reaction is denoted by delta G.
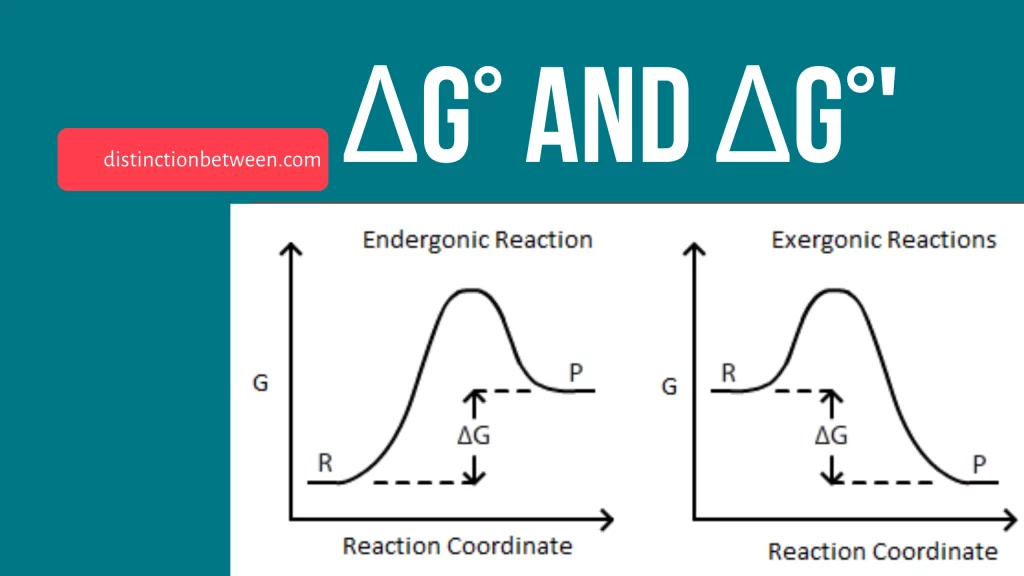
Delta G can be used to predict the spontaneity of a reaction. A reaction is spontaneous if delta G is negative. This means that the reaction will proceed without any external input of energy. A reaction is non-spontaneous if delta G is positive. This means that the reaction will not proceed on its own and requires an external input of energy.
Delta G naught is the change in Gibbs free energy for a reaction at standard conditions. Standard conditions are defined as a temperature of 298K and a pressure of 1 atm. Delta G naught is a constant for a given reaction and does not depend on the concentrations of the reactants and products.
The difference between delta G and delta G naught is the change in Gibbs free energy due to non-standard conditions. For example, if the concentration of a reactant is increased, the change in Gibbs free energy for the reaction will become more negative.
Quick difference between ΔG° and ΔG°’ Table
| Property | ΔG° | ΔG°’ |
|---|---|---|
| Definition | Change in Gibbs free energy at standard conditions (298 K and 1 atm) | Change in Gibbs free energy at standard biological conditions (298 K and 1 atm, pH 7) |
| Symbols | ΔG° | ΔG°’ |
| Standard conditions | 298 K and 1 atm | 298 K, 1 atm, and pH 7 |
| Applications | Predicting the spontaneity of reactions at standard conditions | Predicting the spontaneity of reactions at standard biological conditions |
Examples:
- The standard free energy of formation of water is ΔG°f = -286 kJ/mol. This means that it is spontaneous to form water from hydrogen and oxygen under standard conditions.
- The standard free energy of hydrolysis of ATP is ΔG°’ = -30.5 kJ/mol. This means that it is spontaneous to hydrolyze ATP to ADP and Pi under standard biological conditions.
Note that ΔG°’ is approximately equal to ΔG° at pH 7. However, the difference between the two can be significant for reactions that involve protons, such as acid-base reactions.
Delta G
Delta G can be calculated using the following equation:
delta G = delta H - T * delta S
where:
- delta G is the change in Gibbs free energy
- delta H is the change in enthalpy
- T is the temperature in Kelvin
- delta S is the change in entropy
Delta H is the change in enthalpy for a reaction. It is a measure of the heat released or absorbed during a reaction. Delta S is the change in entropy for a reaction. It is a measure of the disorder of the system.
The sign of delta G can be used to predict the spontaneity of a reaction:
- If delta G is negative, the reaction is spontaneous.
- If delta G is positive, the reaction is non-spontaneous.
- If delta G is zero, the reaction is at equilibrium.
Delta G naught
Delta G naught can be calculated using the following equation:
delta G naught = -RT * ln K
where:
- delta G naught is the change in Gibbs free energy at standard conditions
- R is the gas constant (8.314 J/mol*K)
- T is the temperature in Kelvin
- K is the equilibrium constant
The equilibrium constant is a measure of the extent to which a reaction proceeds at equilibrium. It is calculated by dividing the concentrations of the products by the concentrations of the reactants.
Delta G naught is always negative for spontaneous reactions. This means that the reaction will proceed without any external input of energy. Delta G naught is zero for reactions at equilibrium. This means that the forward and reverse reactions are proceeding at the same rate.
Applications of Delta G and Delta G naught
Delta G and delta G naught are important thermodynamic functions that have a wide range of applications in chemistry and other fields. For example, delta G can be used to:
- Predict the spontaneity of a reaction
- Calculate the equilibrium constant of a reaction
- Design chemical processes
- Understand the thermodynamics of biological systems
Delta G naught can be used to:
- Calculate the equilibrium constant of a reaction
- Determine the feasibility of a reaction
- Compare the thermodynamics of different reactions
Examples
Here are some examples of how delta G and delta G naught can be used:
Predicting the spontaneity of a reaction:
Consider the following reaction:
N2(g) + 3H2(g) -> 2NH3(g)
To predict the spontaneity of this reaction, we need to calculate delta G. We can use the following equation:
delta G = delta H - T * delta S
where:
- delta G is the change in Gibbs free energy
- delta H is the change in enthalpy
- T is the temperature in Kelvin
- delta S is the change in entropy
The change in enthalpy (delta H) for this reaction is -92.2 kJ/mol. The change in entropy (delta S) for this reaction is -199.2 J/mol*K. Assuming a temperature of 298K, we can calculate delta G as follows:
delta G = -92.2 kJ/mol - 298K * (-199.2 J/mol*K)
delta G = -33.3 kJ/mol
Since delta G is negative, the reaction is spontaneous. This means that the reaction will proceed without any external input of energy.
Calculating the equilibrium constant of a reaction:
We can use the following equation to calculate the equilibrium constant (K) for a reaction:
delta G naught = -RT * ln K
where:
- delta G naught is the change in Gibbs free energy at standard conditions
- R is the gas constant (8.314 J/mol*K)
- T is the temperature in Kelvin
- K is the equilibrium constant
Assuming a temperature of 298K and a delta G naught of -33.3 kJ/mol, we can calculate the equilibrium constant as follows:
-33.3 kJ/mol = -8.314 J/mol*K * 298K * ln K
ln K = 1.54
K = e^1.54
K = 4.7
This means that at equilibrium, the ratio of the concentrations of the products to the concentrations of the reactants will be 4.7.
Other examples:
- Delta G can be used to design chemical processes by choosing the conditions that favor the desired product.
- Delta G can be used to understand the thermodynamics of biological systems by predicting the spontaneity of biochemical reactions.
- Delta G naught can be used to compare the thermodynamics of different reactions and to determine the feasibility of a reaction.
Frequently asked questions
Q: What is the difference between delta G and delta G naught?
A: Delta G is the change in Gibbs free energy at any conditions, while delta G naught is the change in Gibbs free energy change at standard conditions. Standard conditions are defined as a temperature of 298K and a pressure of 1 atm.
Q: How can I use delta G to predict the spontaneity of a reaction?
A: If delta G is negative, the reaction is spontaneous. This means that the reaction will proceed without any external input of energy. If delta G is positive, the reaction is non-spontaneous. This means that the reaction will not proceed on its own and requires an external input of energy.
Q: How can I use delta G naught to calculate the equilibrium constant of a reaction?
A: You can use the following equation to calculate the equilibrium constant (K) for a reaction:
delta G naught = -RT * ln K
where:
- delta G naught is the change in Gibbs free energy at standard conditions
- R is the gas constant (8.314 J/mol*K)
- T is the temperature in Kelvin
- K is the equilibrium constant
Conclusion
Delta G and delta G naught are important thermodynamic functions that have a wide range of applications in chemistry and other fields. The Delta G can be used to predict the spontaneity of a reaction, calculate the equilibrium constant of a reaction, design chemical processes, and understand the thermodynamics of biological systems. Delta G naught can be used to calculate the equilibrium constant of a reaction, determine the feasibility of a reaction, and compare the thermodynamics of different reactions.